The global push for electric vehicles (EVs) has placed lithium-ion battery manufacturing under intense scrutiny for quality, consistency, and safety. At the heart of this process lies one critical factor often invisible to the naked eye: particulate contamination.
Setra Blog
July 10, 2025
How Setra Particle Counters Support Precision and Safety in Lithium-Ion Battery Production
July 02, 2025
Keeping It Clean: How Setra Particle Counters Support ISO 14644-1 Compliance in Data Centers
In the fast-paced world of data centers, uptime is everything. Yet, one of the most overlooked threats to server reliability and equipment longevity is also one of the smallest—airborne particulate contamination. Dust, dirt, and microscopic particles can silently degrade performance, disrupt airflow, interfere with circuit boards, and even void equipment warranties. This is where Setra’s advanced particle counters come into play, helping data center operators meet air cleanliness standards, reduce downtime, and maintain the reliability their clients expect.
There are many major benefits to be gained from the implementation of a Setra Continuous Environmental Monitoring System (CEMS). The use cases shown below exemplify the ongoing value delivered through this secure, highly available, cloud-based, real-time environmental monitoring, alarming, reporting and data collection platform.
September 23, 2022
The Value of Environmental Monitoring Systems (EMS) Through Application Programming Interfaces (APIs)
What is a REST API and Why Should I Care?
The awareness and utilization of APIs has exploded in recent years. Originally the domain of the software development community, these powerful interoperability enablers are now at the forefront of web, cloud-based and mobile device application development.
Let’s face facts; manual monitoring of cleanroom particulate counts is costly, monotonous and highly error prone. Cleanroom managers are very aware that by far the greatest contamination threats come from individuals entering controlled spaces. Understandably, to reduce the number people accessing these critical environments, many companies are tasking the cleanroom operations staff already working within these spaces with the additional responsibility for manual environmental monitoring (EM). While this approach has obvious benefits, such as lower labor costs and reduced contamination risks, it also introduces significant new data integrity issues.
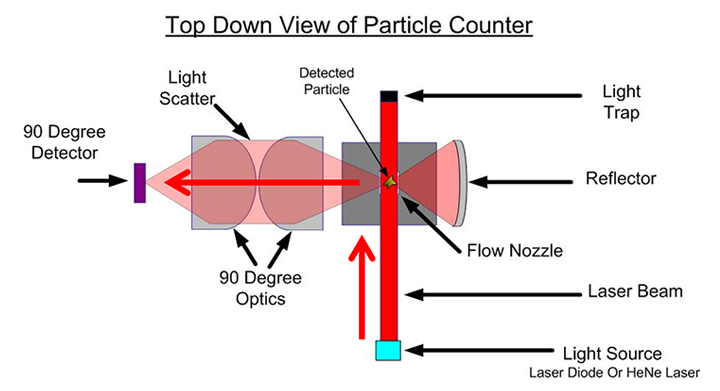
Setra’s wide array of non-viable particle counters are used throughout critical environments to measure particle concentrations of various sizes; these counters use a long-life laser diode, light scattering, and photo detector to calculate particle size and quantities as the sampled air passes through. Particle counters are required for cleanroom certification but are also often used for continuous, sequential, or periodic monitoring of the space between certifications and to ensure that particle levels are maintained below critical thresholds during manufacturing. This post will look at Setra’s different particle counters and their differences to help you decide upon the correct unit(s) for your application.
Setra’s comprehensive range of particle counters and air quality monitors are the most advanced, high-performance instruments available. This industry-leading position has developed from an enduring, intense focus on innovation and original intellectual property creation.
Environmental monitoring, especially particle counting, in cleanrooms is used to show that said cleanroom operates within its set parameters before and during manufacturing processes. The safety and quality of products can be affected if too many particles enter the manufacturing space or process. However, this monitoring is commonly still a manual process in many cleanrooms instead of routine or continuous particle counting. Cleanrooms are highly regulated environments that require the utmost attention to air quality and cleanliness.
To continue operating, a critical environment must meet many safety standards to keep their space free of harmful particles. If an environment is not monitored, it can be easily contaminated by harmful particles such as mold. Air sampling is one of the most common ways to monitor for - and thus prevent - mold. Viable and non-viable air sampling can be employed in detecting mold and other harmful particles in the air. Not monitoring for those particles can lead to disaster.
Subscribe to Our Blog!
Topics
- Critical Environments (182)
- HVAC/R (179)
- General Industrial (153)
- Building Automation (134)
- General Industrial OEM (92)
- Energy Management (85)
- Test and Measurement (66)
- HVAC/R OEM (58)
- Barometric (44)
- Alternative Fuels (42)
- Medical (40)
- Process/Mfg Tank Level (40)
- Water and Wastewater (39)
- OHV (38)
- Oil and Gas (35)
- Industrial Vacuum (29)
- Calibration (25)
- Semiconductor (25)
- Particle Counting (20)
- Cleanroom Monitoring (17)
- Room Pressure Monitoring (16)
- Trade Show (12)
- cleanroom environment (12)
- Scales (11)
- Environmental Monitoring (10)
- Power Monitoring (10)
- Healthcare (9)
- Power Meters (9)
- Software (9)
- cleanroom monitoring systems (9)
- Case Study (8)
- critical environment technologies (8)
- data centers (8)
- Humidity (7)
- particle counter (6)
- pressure transducers (6)
- LITE room pressure monitor (5)
- hardware and software cleanroom monitoring systems (5)
- setra lite (5)
- Compliance (3)
- Video (3)
- hospital spaces (3)
- FAQ & Troubleshooting (2)
- Monitoring Compounding Pharmacies (2)
- Semiconductor Manufacturing (2)
- agencies that monitor pharmacies (2)
- energy (2)
- hvac (2)
- laboratories (2)
- monitor compound pharmacy (2)
- protected environment (2)
- regulatory compliance (2)
- setra lite features (2)
- usp 797 (2)
- Current Sensors and Transducers (1)
- Current Transformers (1)
- Lithium-Ion Battery (1)
- Pressure (1)
- aerospace cleanrooms (1)
- cems (1)
- digital transformation (1)
- ipv6 multicast (1)
- ipv6 multicast address (1)
- ipv6 multicast address range (1)
- isolation room pressure monitoring (1)
- multicast address ipv6 (1)
- multicast ipv6 (1)
- operating room (1)
- pharma 4.0 (1)
- pressure sensor (1)
- pressure transducer companies (1)
- semi conductor (1)
- sensors and transducers (1)
- setra pressure transducers (1)
- submetering (1)
- sustainability (1)
- temperature monitor (1)
- temperature monitoring for pharmacies (1)
- transducers (1)
- usp 800 (1)
- water (1)
- what does hvac stand for (1)
- what is a transducer (1)
- what is hvac (1)