The global push for electric vehicles (EVs) has placed lithium-ion battery manufacturing under intense scrutiny for quality, consistency, and safety. At the heart of this process lies one critical factor often invisible to the naked eye: particulate contamination.
Setra Blog
July 10, 2025
How Setra Particle Counters Support Precision and Safety in Lithium-Ion Battery Production
July 02, 2025
Keeping It Clean: How Setra Particle Counters Support ISO 14644-1 Compliance in Data Centers
In the fast-paced world of data centers, uptime is everything. Yet, one of the most overlooked threats to server reliability and equipment longevity is also one of the smallest—airborne particulate contamination. Dust, dirt, and microscopic particles can silently degrade performance, disrupt airflow, interfere with circuit boards, and even void equipment warranties. This is where Setra’s advanced particle counters come into play, helping data center operators meet air cleanliness standards, reduce downtime, and maintain the reliability their clients expect.
Setra Lite™ has long been a trusted visual pressure indicator, providing clear and immediate pressure status readings for critical environments. Now, with key new upgrades, Setra Lite™ offers even greater convenience, adaptability, and visibility to meet the needs of facility managers, maintenance personnel, and staff.
In HVAC systems, air filters are critical for maintaining clean, efficient, and reliable operation in various applications, including healthcare facilities, laboratories, cleanrooms, data centers, and other critical environments. By ensuring optimal air quality and proper system performance, air filters help protect equipment, processes, and occupants. However, monitoring their status is vital to maximize efficiency and minimize energy costs. Setra's Filter Alert™ offers a state-of-the-art solution for filter health monitoring, providing the precision and simplicity needed to keep HVAC systems running smoothly.
The latest version of Setra EDGE Software is now available! This new software release is packed with new features, bug fixes, and updates to improve performance.
November 01, 2024
The Importance of Differential Pressure Sensor Accuracy and Calibration in Data Centers
Maintaining precise control over environmental conditions is essential for modern data centers, where even minor fluctuations in temperature, humidity, and air quality can impact uptime, operational costs, and efficiency. With the rapid growth of data centers supporting AI, machine learning, and digital infrastructure, reliability is critical. Differential pressure sensors play a central role in sustaining this reliability, and accurate calibration is key to their performance. To meet this demand, Setra Systems has partnered with Standard Calibrations, Inc. (SCI), creating a powerful end-to-end solution that ensures accurate sensor performance across all stages of data center operation.
We are pleased to announce that Setra Systems has joined forces with Standard Calibrations Inc. to transform measurement and control in mission-critical data centers!
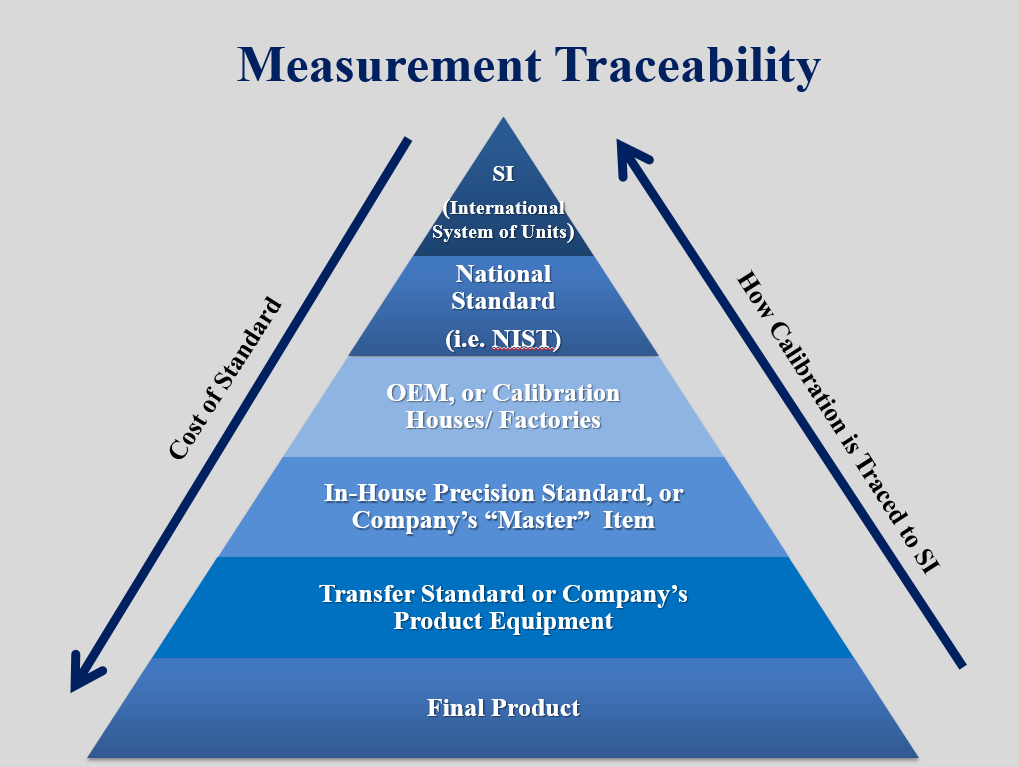
Pressure sensors are used across a variety of industries to provide accurate, real-time data of how a process or system is operating in a given application. But how can you tell if your data is accurate? The most common method to ensure the accuracy of sensor output is to calibrate your sensor against a higher accuracy pressure sensor (calibration standard). Now, how can you tell if your calibration standard is accurate? This is where measurement traceability plays into the calibration process.
A dynamometer, or "dyno" for short, is a device for measuring force, moment of force (torque), or power. For example, the power produced by an engine, motor or other rotating prime mover can be calculated by simultaneously measuring torque and rotational speed (rpm).
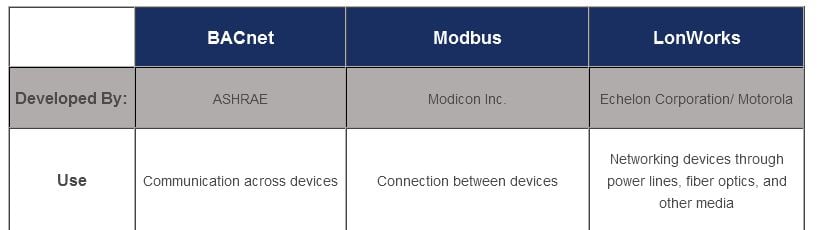
Until recently, there was no standard industry network protocol for building automation, and users had to choose between many different systems from different manufacturers. Proprietary communications were a result of no off-the shelf communication solution.
Today, we have reached a place where there are three inter-operable standard network protocols to choose from: BACnet, LonWorks and Modbus. All three are widely used, and according to a Building Operating Management survey in 2011, 62% of respondents had at least one BACnet application; for LonWorks the percentage was 40%, while for Modbus the number was 30%.
Subscribe to Our Blog!
Topics
- Critical Environments (182)
- HVAC/R (179)
- General Industrial (153)
- Building Automation (134)
- General Industrial OEM (92)
- Energy Management (85)
- Test and Measurement (66)
- HVAC/R OEM (58)
- Barometric (44)
- Alternative Fuels (42)
- Medical (40)
- Process/Mfg Tank Level (40)
- Water and Wastewater (39)
- OHV (38)
- Oil and Gas (35)
- Industrial Vacuum (29)
- Calibration (25)
- Semiconductor (25)
- Particle Counting (20)
- Cleanroom Monitoring (17)
- Room Pressure Monitoring (16)
- Trade Show (12)
- cleanroom environment (12)
- Scales (11)
- Environmental Monitoring (10)
- Power Monitoring (10)
- Healthcare (9)
- Power Meters (9)
- Software (9)
- cleanroom monitoring systems (9)
- Case Study (8)
- critical environment technologies (8)
- data centers (8)
- Humidity (7)
- particle counter (6)
- pressure transducers (6)
- LITE room pressure monitor (5)
- hardware and software cleanroom monitoring systems (5)
- setra lite (5)
- Compliance (3)
- Video (3)
- hospital spaces (3)
- FAQ & Troubleshooting (2)
- Monitoring Compounding Pharmacies (2)
- Semiconductor Manufacturing (2)
- agencies that monitor pharmacies (2)
- energy (2)
- hvac (2)
- laboratories (2)
- monitor compound pharmacy (2)
- protected environment (2)
- regulatory compliance (2)
- setra lite features (2)
- usp 797 (2)
- Current Sensors and Transducers (1)
- Current Transformers (1)
- Lithium-Ion Battery (1)
- Pressure (1)
- aerospace cleanrooms (1)
- cems (1)
- digital transformation (1)
- ipv6 multicast (1)
- ipv6 multicast address (1)
- ipv6 multicast address range (1)
- isolation room pressure monitoring (1)
- multicast address ipv6 (1)
- multicast ipv6 (1)
- operating room (1)
- pharma 4.0 (1)
- pressure sensor (1)
- pressure transducer companies (1)
- semi conductor (1)
- sensors and transducers (1)
- setra pressure transducers (1)
- submetering (1)
- sustainability (1)
- temperature monitor (1)
- temperature monitoring for pharmacies (1)
- transducers (1)
- usp 800 (1)
- water (1)
- what does hvac stand for (1)
- what is a transducer (1)
- what is hvac (1)