In HVAC/R applications it is helpful to understand the methods used to determine air velocity. Air velocity (distance traveled per unit of time) is most often expressed in feet per minute (FPM). Multiplying air velocity by the area of a duct allows you to determine the air volume flowing past a point in the duct per unit of time. Volume flow is generally measured in cubic feet per minute (CFM).
Setra Blog
Setra's MR Series features a lineup of cutting-edge pressure transducers designed for HVAC applications. From the adaptable MR1 and MR2 to the high-performance MRG and versatile MRC, each product in this series combines multi-range capabilities with top-tier performance. In this article, we'll delve into the features and advantages of these sensors, showcasing why they're the go-to choice for contractors looking for reliability, flexibility, and precision in their HVAC applications.
The Setra MR1 is the ideal product for any contractor, combining the flexibility of a multi-range with the performance of a single range sensor. The MR1 has 8 selectable ranges (0.1, 0.25, 0.5, and 1.0” W.C.) and 3 selectable outputs, easily adjustable on the job with a flip of a switch or jumper. The MR1 uses an IP67/NEMA 4-rated housing and has a conduit fitting for easy wiring, making the MR1 an ideal solution for any general HVAC application.
The Setra MR2 stands out as the perfect choice for contractors, merging the versatility of a multi-range sensor with the efficiency of a single range sensor. With 8 selectable ranges (1.0, 2.5, 5.0, and 10” W.C.) and 3 selectable outputs, it's effortlessly adaptable on-site with a simple switch or jumper adjustment. Encased in an IP67/NEMA 4-rated housing and featuring a conduit fitting for straightforward wiring, the MR2 is an excellent fit for various general HVAC applications.
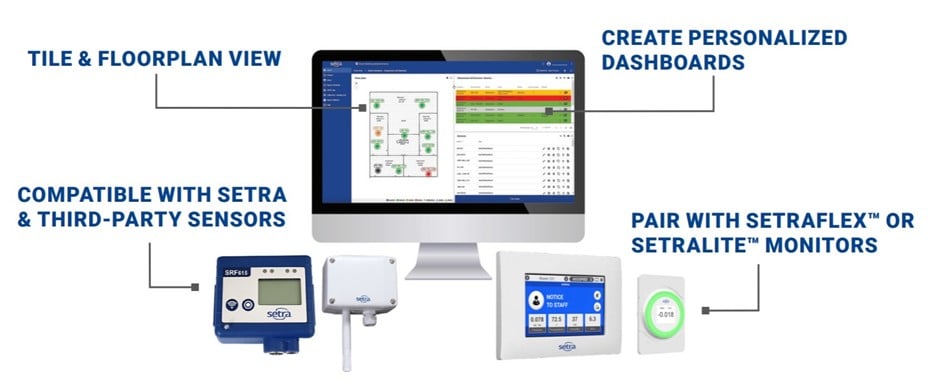
The latest version of Setra CEMS™ Software 6.0 is now available! This new software release is packed with new features and updates to improve the monitoring of critical environment applications.
Discover the complex and ever changing history of room pressure monitoring, a testament to healthcare's commitment to safety. From ancient methods using smoke and feathers to today's sophisticated digital systems, explore how innovation has elevated infection control.
September 18, 2023
From Manual Checks to Money-Saving: How Setra LITE Reshaped Room Pressure Management in Southern California
This case study will explain how a prominent hospital in Southern California successfully improved compliance adherence, patient safety, and hospital efficiency while also seeing significant cost savings by automating room pressure monitoring in critical areas.
July 18, 2023
Maximizing Cost Savings and Increasing Labor Productivity with Setra Lite Room Pressure Monitor
In the fast-paced healthcare environment, optimizing efficiency and reducing costs are paramount objectives for hospitals. The Setra Lite Room Pressure Monitor offers a cutting-edge solution that streamlines monitoring processes, frees up valuable time, and ensures continuous compliance. We delve into the benefits of Setra Lite, highlighting its time-saving features, simplified setup, significant cost savings, and the ability to maintain continuous monitoring.
Enhancing Safety and Compliance
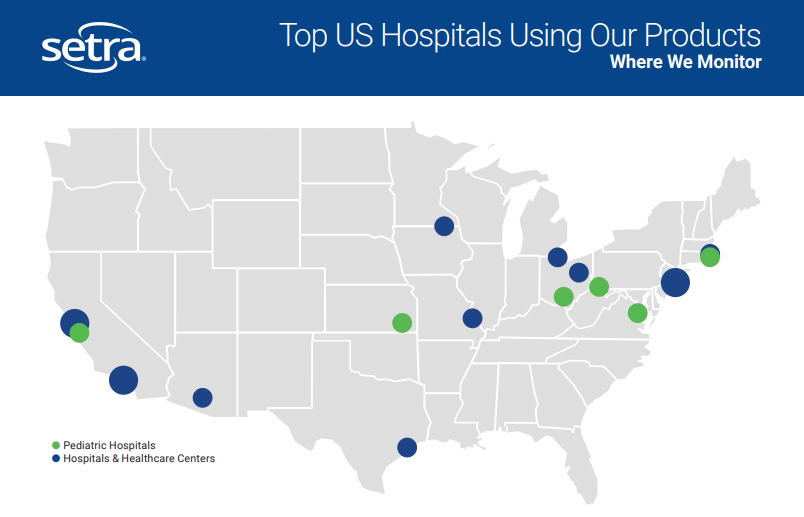
By incorporating Setra's room monitoring solutions, hospitals can proactively address compliance issues in non-critical spaces (such as linen storage, emergency departments, Bronchoscopy Spaces, etc.), ensuring patient safety, staff well-being, and regulatory adherence. Regular monitoring with Setra's reliable and user-friendly solutions minimizes the risk of non-compliance surprises and supports hospitals in achieving successful audits and inspections. This is why Setra's room monitors are trusted by the top 6 cardiology and heart surgery hospitals across the nation.
Isolation spaces represent some of the most critical spaces within a healthcare facility, given the potential harm they can pose to patients and staff if not monitored correctly. These spaces are commonly used to provide care for individuals with compromised immune systems, such as cancer or HIV patients, as well as to safeguard healthcare providers and the public against highly contagious diseases like Tuberculosis and COVID-19. The increased risk of infection from airborne pathogens underscores the importance of maintaining the highest standards of monitoring and equipment reliability. Setra's dependable solutions, trusted by top 5 nationally ranked hospitals across the US, provide the necessary assurance and support to ensure the safety and well-being of patients and healthcare personnel.
June 13, 2023
Compounding Pharmacies: The Importance of Setra Room Monitors for Safety and Compliance
Ensure USP <797> and <800> Compliance
With Setra's trusted room monitors, compounding pharmacies can significantly enhance safety measures and meet the stringent requirements outlined by USP <800> and USP <797> standards. Compliance not only safeguards the well-being of pharmacy personnel but also prevents contamination of medications. Setra's reputation for reliability is exemplified by being trusted in 13 of the 20 top-rated hospitals in the US*. By leveraging Setra's proven monitoring solutions, compounding pharmacies can effectively mitigate risk, prioritize safety, and ensure compliance with regulatory standards.
Are You Compliant With USP 797 and USP 800 Standards?
Revised standards for USP General Chapter <797> for compounded sterile preparation (CSP) have been finalized and are active as of Nov 1, 2023. These revisions will also affect USP <800> where hazardous drug (HD) compounding is applicable. Both standards include environmental monitoring and management requirements that were not previously enforced and must be met to achieve accreditation through local and national governing bodies. Understanding the conditions and monitoring requirements is critical in ensuring compliance.
If you need help understanding the UPS <797> and USP <800> requirements and want to find compliant solutions, we would be happy to assist! Contact Setra today to talk with our product team and make sure that all of your spaces are compliant.
Subscribe to Our Blog!
Topics
- Critical Environments (182)
- HVAC/R (179)
- General Industrial (153)
- Building Automation (134)
- General Industrial OEM (92)
- Energy Management (85)
- Test and Measurement (66)
- HVAC/R OEM (58)
- Barometric (44)
- Alternative Fuels (42)
- Medical (40)
- Process/Mfg Tank Level (40)
- Water and Wastewater (39)
- OHV (38)
- Oil and Gas (35)
- Industrial Vacuum (29)
- Calibration (25)
- Semiconductor (25)
- Particle Counting (20)
- Cleanroom Monitoring (17)
- Room Pressure Monitoring (16)
- Trade Show (12)
- cleanroom environment (12)
- Scales (11)
- Environmental Monitoring (10)
- Power Monitoring (10)
- Healthcare (9)
- Power Meters (9)
- Software (9)
- cleanroom monitoring systems (9)
- Case Study (8)
- critical environment technologies (8)
- data centers (8)
- Humidity (7)
- particle counter (6)
- pressure transducers (6)
- LITE room pressure monitor (5)
- hardware and software cleanroom monitoring systems (5)
- setra lite (5)
- Compliance (3)
- Video (3)
- hospital spaces (3)
- FAQ & Troubleshooting (2)
- Monitoring Compounding Pharmacies (2)
- Semiconductor Manufacturing (2)
- agencies that monitor pharmacies (2)
- energy (2)
- hvac (2)
- laboratories (2)
- monitor compound pharmacy (2)
- protected environment (2)
- regulatory compliance (2)
- setra lite features (2)
- usp 797 (2)
- Current Sensors and Transducers (1)
- Current Transformers (1)
- Lithium-Ion Battery (1)
- Pressure (1)
- aerospace cleanrooms (1)
- cems (1)
- digital transformation (1)
- ipv6 multicast (1)
- ipv6 multicast address (1)
- ipv6 multicast address range (1)
- isolation room pressure monitoring (1)
- multicast address ipv6 (1)
- multicast ipv6 (1)
- operating room (1)
- pharma 4.0 (1)
- pressure sensor (1)
- pressure transducer companies (1)
- semi conductor (1)
- sensors and transducers (1)
- setra pressure transducers (1)
- submetering (1)
- sustainability (1)
- temperature monitor (1)
- temperature monitoring for pharmacies (1)
- transducers (1)
- usp 800 (1)
- water (1)
- what does hvac stand for (1)
- what is a transducer (1)
- what is hvac (1)