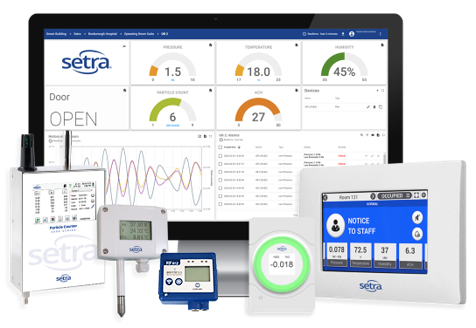
Are You Compliant With USP 797 and USP 800 Standards?
Revised standards for USP General Chapter <797> for compounded sterile preparation (CSP) have been finalized and are active as of Nov 1, 2023. These revisions will also affect USP <800> where hazardous drug (HD) compounding is applicable. Both standards include environmental monitoring and management requirements that were not previously enforced and must be met to achieve accreditation through local and national governing bodies. Understanding the conditions and monitoring requirements is critical in ensuring compliance.
If you need help understanding the UPS <797> and USP <800> requirements and want to find compliant solutions, we would be happy to assist! Contact Setra today to talk with our product team and make sure that all of your spaces are compliant.