Hospital pharmacies are unique because of the critical role they play in maintaining patient health and safety. Pharmacies that perform compounded sterile preparations (CSPs), in particular, must meet specific clean room standards to ensure sterile conditions are maintained. While there are many established environmental requirements in these spaces, some pharmacy managers are choosing to go the extra mile in exchange for added peace of mind.
ISO and USP classifications
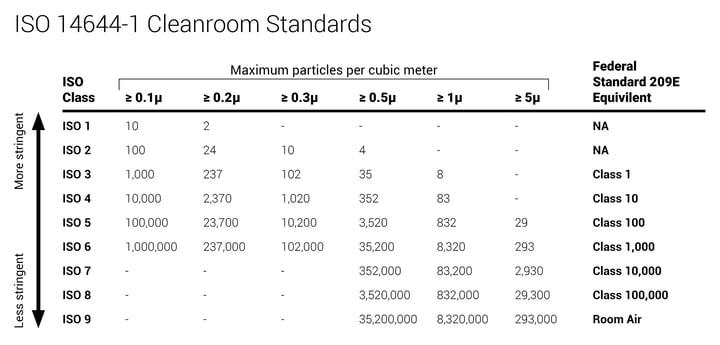
In the United States, compounding pharmacies follow the guidelines defined by the United States Pharmacopeia (USP) organization. These environmental guidelines are known as USP 797 and USP 800 and they regulate how hospitals operate these types of facilities. Clean room conditions for CSP pharmacies, as specified by the USP, fall into several ISO Classes depending on the area of the pharmacy and what procedures are taking place. One of the most crucial parameters defined here is the density of non-viable particulates.
![]()
Gowning rooms (or anterooms) fall into ISO Class 8. Primary compounding rooms on the other side of an anteroom or ante area need to comply with particulate counts of ISO Class 7. Compounding hoods, bio-safety cabinets, or laminar workflow benches should meet ISO Class 5. To achieve these levels, engineered environmental systems control HVAC with proper air filtration and room pressurization with continuous airflow monitoring in place to meet USP requirements.
Are semi-annual inspections enough?
Day in and day out, personnel are entering and exiting CSP pharmacies to meet patient needs. Supplies are brought in, gowning and de-gowning takes place, and general movement of people and materials are important considerations as it relates to airborne particulate count in each ISO Class area.
"But how does one know if particulate count is within the guidelines during the other 363 days of the year?"
USP guidelines state “Air sampling shall be performed at least semi-annually (i.e., every 6 months) as part of the re-certification of facilities and equipment.” This certification is usually done by a third-party service company. But how does one know if particulate count is within the guidelines during the other 363 days of the year?
What can continuous particle counter tell you?
While many facilities continuously monitor for viable contaminants (i.e. airborne bacteria and viruses), continuous non-viable particle counting is not yet common practice in the majority of hospital pharmacies. Most pharmacists know, however, that non-viable particles often act as vehicles for those other contaminants and aid in their propagation.
"A measurable spike in particle counts can lead to the discovery issues such as a gap in a door seal or a malfunctioning piece of air handling equipment."
Continuous particle counting, with properly set alarms, can also indicate potential issues within an otherwise sterile processing environment. A measurable spike in particle counts can lead to the discovery issues such as a gap in a door seal or a malfunctioning piece of air handling equipment. Additionally, the continuous logging of particle counting data can help exonerate a compliant pharmacy in the case of an incident investigation - ensuring yet another level of confidence about the air quality in your facility.
When patient safety and pharmacy production is on the line, can you afford to rely on the bare minimum semi-annual inspection?